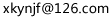反应N2+3H2=2NH3在密闭容器中进行达到平衡,平衡时c(N2)=3.5mol/L,c(H2)=1MOL/L,c(NH3)=5MOL/L
(1)设N2与H2发开始浓度分别为x、y,转化为n,则 N2(g)+3H2(g)?2NH3(g),开始 x y 0转化 n 3n 2n平衡3.5 1 52n=5,解得n=2.5,所以x=3.5+2.5=6mol/L,y=1+3×2.5=8.8mol/L,答:N2和H2的起始浓度分别为6mol?L-1、8.5mol?L-1;(2)N2的转化率为2.5mol/L6mol/L×1005=41.67%,答:N2的转化率为41.67%.
反应N2+3H2=2NH3在密闭容器中进行达到平衡,平衡时c(N2)=3.5mol/L,c(H2)=11MOL/L,c(NH3)=5MOL/L.求: 1.N2和H2的起始浓度。 用等效平衡算 开始浓度:c(N2)=6MOL/L c(N2)=18.5MOL/L N2+3H2=2NH3 能反应出c(NH3)=5MOL/L 那么原来就需要2.5MOL/L的N2 加上平衡时的3.5MOL/L就是c开始(N2)=6MOL/L H2同理 原来需要7.5MOL/L 家上平衡的11就是c开始(H2)=18.5MOL/L 2.N2的转化率 41.67%(5/12) N2开始的时候的浓度是6MOL/L反应转化了的浓度2.5MOL/L 那么2.5/6*100%就是转化率 就大约是41.67% 3.平衡时压强为开始时压强得?% 由气体对气壁的压强定义 条件相同时(温度压强)体积相同的容器的气体压强比等于两容器中的气体物质的量比 反应开始时有气体重物质的量18.5+6=24.5MOL 反应后3.5+11+5=19.5MOL 那么19.5/24.5*100%就是所求大约等于79.59% 4.平衡时NH3占总体积的体积分数 由阿佛加得罗定理 同条件下(温度 压强)的物质的量比等于气体的体积比 那么反应后一共有气体19.5MOL NH3有5MOL 那么NH3的体积分数就等于n(NH3)/n(总)=5/19.5*100%≈25.64% 明白了么? 祝进步 歆蟹
1.初始c(N2)=6mol/L 【计算 5/2+3.5】 c(H2)=8.5mol/L 【计算(5/2)*3+1】2. (6-3.5)/6=41.67%
3.(3.5+1+5)/(6+8.5)=65.52%
4 5/(5+1+3.5)=52.63%
达到化学平衡时生成的氨气所需要消耗的H2和N2的量分别为:
体积恒定,物质的量直接转化为浓度计算
c(N2)=5/2=2.5mol/L
c(H2)=(3*5)/2=7.5mol/L
所以初始浓度为:
c(N2)=3.5+2.5=6mol/L
c(H2)=11+7.5=18.5mol/L
N2的转化率=消耗的N2/初始的N2=2.5/6=41.67%
由于体积恒定,压强比等于体积比,
P平/P初=V平/V初=(3.5+11+5)/(6+18.5)=75.6%
NH3占总体积的体积分数为:
5/(3.5+11+5)=25.6%
K=4*4/(3*9*9*9)=0.007316 C(N2)=3 4/2=5mol/L C(H2)=9 4/2*3=15mol/L
JFG290
□ Hui Shanghai newspaper correspondent reported
latest Peoples Bank of China "in November 2009 the operation of financial markets" report,dunk high heels, 09 the first 11 months of total inter-bank bond market, issuance of bonds (excluding central bank bills) 3.84137 trillion yuan, surged 84.6% year on year , in which the chain to achieve the previous month in November, 55.9% increase. Analysts said that in the proactive fiscal policy and loose monetary policy orientation, the 2009 bond market has achieved rapid expansion, while in 2010 the total size of new debt supply will remain high.
stable inter-bank bond market supply and demand
"2009 Nian 11 Yue Fen operation of financial markets" shows that eleven months of 2009,king griffey shoes, total inter-bank bond market,jordan heels, bonds 3.84137 trillion yuan, up 84.6% significant increase; the November issue of the size of the previous month increased 55.9% the month inter-bank bond market,nike high heels uk, bonds 489.61 billion yuan. In managed volume, the end of November,nike high heels, the amount of the bond market managed to 13.17 trillion yuan (excluding central bank bills), which hosted the inter-bank bond market volume of 12.89 trillion yuan, accounting for the amount of the bond market managed 97.9%,jordan 4 high heels, debt exchange City hosted the total of only 280 billion yuan.
in period, the first 11 months of 2009, the inter-bank bond market, bonds issued to the following 5-year bonds based. But in November, the inter-bank bond market,nike dunk heels, the following 5-year bonds increased significantly the proportion of the previous month.
significant increase in the supply of new debt, while market funds face near the end of the period, still continues this year, the liberal state. November statistics show that lending transactions, although the supply of new debt in November more than half of the chain theres a substantial increase, but overall the month due to market liquidity abundant, market interest rates stable in overall down slightly. The weighted average interbank interest rate in November was 1.25%, compared with October, down 5 basis points; 7 days weighted average interest rate of 1.46%, compared to 10 fell 7 basis points.
2010 years or continued high expansion
12 27, Premier Wen Jiabao told Xinhua News Agency interview the next year once again highlighted the main economic policy measures,nike dunk heels, the continuity and stability, which market participants said that the supply of bonds in 2010 will continue high. Before that,jordan shoes for women, some institutions have also been on view next years bond issue for a more adequate estimate. CITIC Securities is expected next year the size of newly issued bonds in the 4.6-4.9 trillion yuan; Guohai expected data was 4.81 trillion yuan; and Guoxin Securities, forecast full-year bond issuance next year will reach 5.06 trillion yuan.
sub-species,nike shoes uk, the market for treasury bonds next year still have high expectations. Sealand Securities expects fiscal 2010 budget deficit will remain at 8,000 billion, and next year maturity government debt of about 750 billion yuan treasury bonds next year will remain so up to 1.55 trillion yuan,new jordan heels, almost equal in 2009. In the financial bonds,king griffey shoes, the market forecast financial bonds issued next year will total 1.3 trillion yuan to 1.6 trillion yuan between, representing about 1.3 trillion yuan this year,ken griffey shoes, there is a certain level of increase. Sealand Securities predicted that next year corporate bonds and corporate debt issue size will grow 20% over the current year,dunk heels, reaching 550 billion yuan, short trading and will issue medium-term notes totaling approximately 1.15 trillion yuan.
the impact on the market,griffey shoes sale, some market participants said that the dominant factor in the market next year may not be mainly due to the supply of new debt, inflation and base interest rates expected to go into the bond market will be the main reason for the decision.
has _COUNT_ comments I want to comment . CorrTxt_01 {border-top: 1px dashed # C8D8F2; margin-top:-1px;}. CorrTxt_01 h3 {font-weight: bold; padding: 5px 0 0 3px; line-height: 25px; margin: 0;}. CorrTxt_01 ul {padding: 0 0 20px 18px;}. CorrTxt_01 ul li {font-size: 14px; line-height: 164.28%;}
已知合成氨反应为:N2+3H2?2NH3,在一定温度下,向2L密闭容器中,加入2m...
答:向2L密闭容器中,加入2molN2和5molH2,一定条件下使之反应,经过2min后测得NH3为0.4mol,则 N2 +3H2 =2 NH3,起始量(mol)2 5 0变化量(mol)0.2 0.6 0.4平衡量(mol)1.8 4.4 0.4(1)用NH3表示这2min内该反应的反应速率=0.4mol2L2min=0.1mol/L?min...
对于反应N2+3H2生成2NH3请问在恒温恒容密封容器内充入He
答:因为每种物质的浓度=该物质的物质的量/容器容积,所以,c(N2),c(H2),c(NH3)都不变化。而正反应速率与反应物浓度c(N2),c(H2)有关,c(N2),c(H2)不变,正反应速率就不变;逆反应速率与生成物浓度c(NH3)有关,c(NH3)不变,逆反应速率就不变。2、压强对化学反应速率的影响,实质是通过改变...
在密闭容器中,保持一定温度进行如下反应N2+3H2=2NH3,已经加入1molN2和3...
答:(1)写出该反应的化学平衡常数表达式。K=C(NH3)^2/[C(N2)*C(H2)^3](2)a与b的关系是:a > b(填<>=)
...发生如下反应:N2+3H2=2NH3,反应达到平衡后,向容器中通入氦气。_百度...
答:恒压充入氦气,体积变大,相当于同时稀释N2、H2、NH3浓度,平衡逆向移动。
在密闭容器中进行下述反应:N2+3H2=2NH3,反应开始时,N2 H2 NH3的浓度...
答:选D 假设N2和H2全部反应完,NH3最多也就0.4mol,3不对 假设NH3全部分解成N2和H2,N2最多也就0.2mol,1不对 所以排除A,B,C
温度一定。可逆反应N2+3H2=2NH3在的密封容器中进行!开始加入2mol氮气和...
答:此反应为消耗1molN2、3molH2,生成2molNH3,假设氢气耗尽,则最多2摩尔氨气;最少不反应,0摩尔氨气。所以氨气浓度范围是0—2摩尔。
高中化学 N2+3H2=2NH3反应达到平衡时三者浓度比为1:3:2,在想容器中按...
答:1、平衡时再向容器中按物质量1:3:2通入三者,压强增大,平衡向气体体积减小的方向移动。即向右移动,所以NH3的体积分数(增大)。2、 2SO2 + O2 = 2SO3 初始: 0.23 0.11 0 过程: 2x x 2x 平衡:0.23-2x 0.11-x 2x 得到0.12molSO3,所以x...
在2L容器中发生反应N2+3H2=(可逆)2NH3,经一段时间后NH3
答:设时间为t秒,则在这段时间内氢气增加的物质的量为0.6mol/L / S*2L*tS=1.2tmol,根据化学方程式:3H2~~~2NH3 3 2 1.2tmol 0.8tmol 而这段时间NH3的物质的量增加了2.4mol 所以t=2.4mol /0.8mol=3秒
在N2+3H2=2NH3反应中恒压条件下充入H2,平衡向哪个方向移动
答:向右移动。在N2+3H2=2NH3反应中恒压条件下充入H2,所以H2的浓度增大,为了到达平衡,所以反应向右移动
反应N2+3H2=2NH3.∧H=-92.4kJ/mol.在密闭容器中进行并达到平衡,最初c...
答:---N2+3H2=2NH3 起始 480 转化 0.4 1.2 0.8 平衡 3.6 6.8 0.8 (1)达到平衡时,N2. H2和NH3的浓度分别是3.6mol/L,6.8mol/L,0.8mol/L (2):平衡时NH3占总体积百分数=0.8/(3.6+6.8+0.8)=7.1